ISO Registration Demystified:
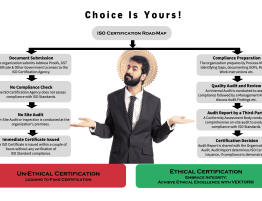
ISO Registration or ISO Certification are the terms used for the same objective, which is to achieve endorsement against requirements of the ISO Standards. ISO has many standards, almost more than 22800. The most commonly known standard is ISO 9001:2015. This is the only Contractual Standard among the ISO 9000 series/ family. Which Means, that registration as well as certification can be granted against this standard under a contract between the Conformity Assessment Body and the organization seeking endorsement. The other supporting non-contractual standards within the ISO 9000 family are ISO 9000 & ISO 9004.
ISO Registration Documents required:
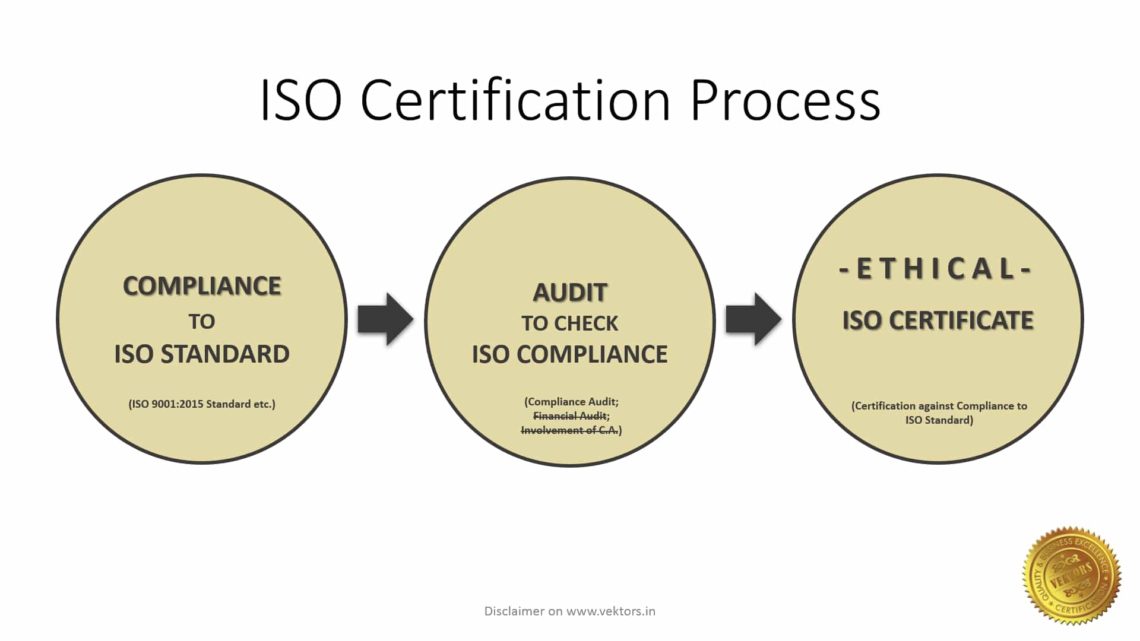
The documents required for ISO Registration against ISO 9001:2015 or ISO 9001:2015 Certification are the documental evidences which are required to prove a part of the compliance against a specific ISO standard. Documental evidences alone are not sufficient to fulfil the requirement of compliance against a specific ISO standard.
Since there are so many published ISO standards applicable for a range of management systems of a variety of industries and sectors, we can only discuss the requirement of a specific standard as the requirement varies from one to another. A few popular ISO standards are ISO 9001:2015, ISO 14001:2015, ISO 13485:2016, ISO 22000:2005, ISO 27001:2013 etc. The ISO management system standards categorized by sector, are as follows:
- Quality
- Industry
- Safety and Security
- General management
- Health and Medical
- Environment and Energy
- Information Technology
- Services
Let’s discuss the documents required for certification against a specific and most commonly used ISO standard, ISO 9001:2015.
So, what are the requirements of ISO 9001:2015 Certification?
However, we cannot arrive at the exact number and type of documentation needed for a specific organization as it varies from organization to organization depending on the scope of services, applicability of clauses and their niche area of concern & requirement etc. Therefore, we can only broadly discuss the most tentative set of documents, in general.
ISO 9001:2015, which is also called as ‘Quality Management System’ or simply QMS, has requirements to establish a Process Dependent Management System (PDMS). This can be established by implementing, documenting, maintaining and effectively monitoring the management system, as per the clause-wise requirements mentioned in the ISO 9001:2015 standard.
A tentative range of documents which an organization may need to exhibit their compliance during an ISO 9001:2015 audit, as categorized under Mandatory documents, Mandatory records and Non-mandatory documents, are as follows:
Mandatory documents:
- Scope of the Quality Management System (QMS) (clause 4.3)
- Quality policy (clause 5.2)
- Quality objectives (clause 6.2)
- Criteria for evaluation and selection of suppliers (clause 8.4.1)
Please note that records marked with * as mentioned below, are only mandatory in cases when the relevant clause is not excluded from the scope of the QMS:
Mandatory records:
- Monitoring and measuring equipment calibration records* (clause 7.1.5.1)
- Records of training, skills, experience and qualifications (clause 7.2)
- Product/service requirements review records (clause 8.2.3.2)
- Record about design and development outputs review* (clause 8.3.2)
- Records about design and development inputs* (clause 8.3.3)
- Records of design and development controls* (clause 8.3.4)
- Records of design and development outputs *(clause 8.3.5)
- Design and development changes records* (clause 8.3.6)
- Characteristics of product to be produced and service to be provided (clause 8.5.1)
- Records about customer property (clause 8.5.3)
- Production/service provision change control records (clause 8.5.6)
- Record of conformity of product/service with acceptance criteria (clause 8.6)
- Record of nonconforming outputs (clause 8.7.2)
- Monitoring and measurement results (clause 9.1.1)
- Internal audit program (clause 9.2)
- Results of internal audits (clause 9.2)
- Results of the management review (clause 9.3)
- Results of corrective actions (clause 10.1)
Non-mandatory documents:
There are many non-mandatory documents that may be used for ISO 9001implementation as the list is exhaustive. However, there is a general consensus among ISO consultants at Mumbai & various quality interpreters across the globe, that following non-mandatory documents are most commonly used:
- Procedure for determining context of the organization and interested parties (clauses 4.1 and 4.2)
- Procedure for addressing risks and opportunities (clause 6.1)
- Procedure for competence, training and awareness (clauses 7.1.2, 7.2 and 7.3)
- Procedure for equipment maintenance and measuring equipment (clause 7.1.5)
- Procedure for document and record control (clause 7.5)
- Sales procedure (clause 8.2)
- Procedure for design and development (clause 8.3)
- Procedure for production and service provision (clause 8.5)
- Warehousing procedure (clause 8.5.4)
- Procedure for management of nonconformities and corrective actions (clauses 8.7 and 10.2)
- Procedure for monitoring customer satisfaction (clause 9.1.2)
- Procedure for internal audit (clause 9.2)
- Procedure for management review (clause 9.3)
Know more? What are the documents required for ISO Certification?
Know more? What is the actual cost of ISO certification?
Know more? How to get ISO Certified in India?